Preview our MCAT-like sample questions and detailed answer explanations. Then, create custom practice tests in the QBank to gauge your exam readiness.
Off Set JS Code
Try a free sample question from each MCAT subject. Once you experience our detailed answer explanations, you’ll understand why our QBank was voted the #1 most helpful MCAT prep resource by students like you!
Biochemistry Behavioral Sciences General Chemistry Organic Chemistry BiochemistryBiochemistry makes up 25% of both the Chemical and Physical Foundations of Biological Systems and the Biological and Biochemical Foundations of Living Systems section of the MCAT. These questions will largely be at the skill level of a first-semester biochemistry course. By learning these concepts, you will have a fuller idea of how biochemical functions support health or can lead to disease. Try the free Biochemistry sample question below:
Acetylation of lysine residues in histones increases gene expression because:
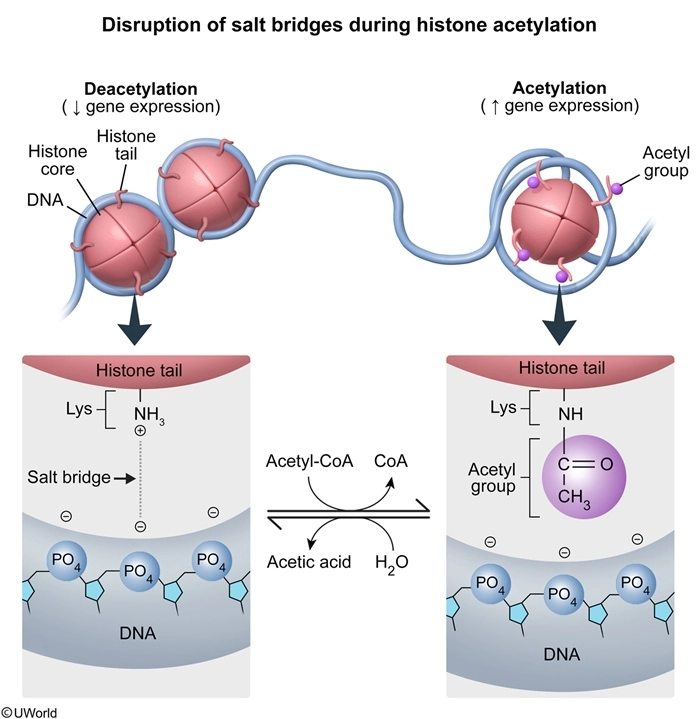
DNA winds tightly around proteins referred to as histones to form structural units known as nucleosomes. Gene expression depends partially on the association of histones with DNA. Genes that are actively transcribed are found on unwound stretches of chromatin called euchromatin, which transcription machinery can easily access. Inactive genes are usually in highly condensed DNA known as heterochromatin, which is much less accessible. Covalent post-translational modifications such as methylation, acetylation, and phosphorylation alter the association of histones with DNA.
DNA is predominantly negatively charged due to the phosphate groups on the backbone. Histones associate with DNA by forming salt bridges between positively charged amino acid residues and negatively charged phosphate groups. These ionic interactions allow histones to bind tightly to DNA and prevent genes from being transcribed. Acetylation of histones involves the transfer of acetyl groups from acetyl coenzyme A to positively charged amino groups on lysine or arginine residues. This modification disrupts salt bridges by reducing the positive charge on histones, which allows DNA to unwind and become more accessible to transcription machinery. As a result, the acetylation of histones causes nucleosomes to relax and increases gene expression.
(Choice A) Negatively charged amino acids (aspartate and glutamate) would be repelled by the negatively charged phosphate groups on DNA.
(Choice B) The carboxyl oxygen atoms in the acetyl groups form hydrogen bonds with water rather than the nitrogenous bases on DNA. Acetylation neutralizes the positive charge on histones and decreases their interaction with DNA.
(Choice D) Lysine residues are positively charged, and associate with negatively charged phosphate groups in DNA. If phosphate groups were positively charged, they would repel lysine.
Educational objective:
Histones associate with DNA by forming salt bridges between their positively charged residues and negatively charged phosphate groups on DNA. Acetylation of histones involves the transfer of an acetyl group to positively charged amino groups on lysine or arginine residues, increasing gene expression by disrupting salt bridges between histones and DNA.
The Behavioral Sciences (Psychology and Sociology) content of the MCAT covers psychological, social, and biological foundations of behavior. In learning these concepts and how to apply them, you will be better able to address the psychological and social aspects of medicine and understand how behaviors impact health. Try the free Behavioral Science practice question below:
Which of the following population pyramids depicts the population that is most likely to decrease in size in the near future?
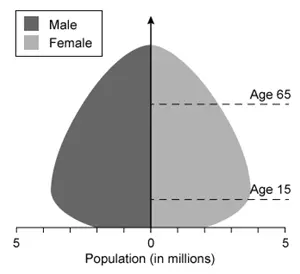
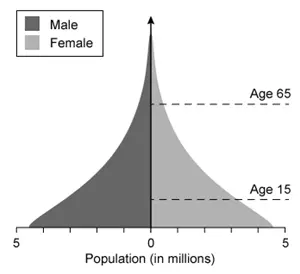
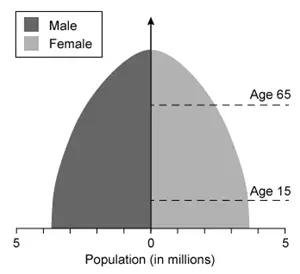
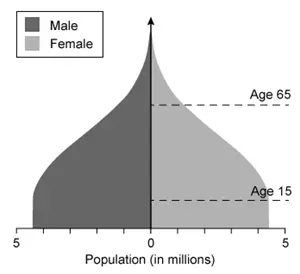
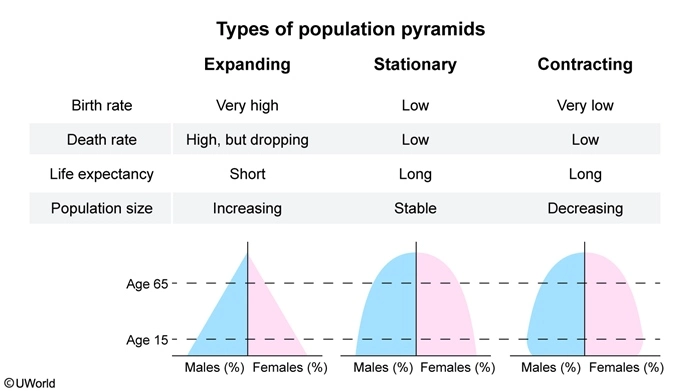
Population pyramids, which display the relative number of males and females in each age cohort in a given population, have three general shapes:
A contracting population pyramid reflects a population with fewer people in the younger age cohorts and more people in the older age cohorts (ie, base is narrower than upper levels of pyramid). In contracting populations, the number of individuals born will not replace the number of older individuals who die, resulting in an overall decrease in population size.
(Choice B) A pyramid with a broad base and narrow top depicts an expanding population that is growing in size.
(Choice C) A pyramid with a broad base and top depicts a stationary population that is relatively stable in size.
(Choice D) A pyramid that is between an expanding and stationary shape depicts a population that was expanding but is in the process of becoming stationary. In this type of population, the growth rate slows, but the overall size of the population does not decrease because more people live to old age.
Educational objective:
A population pyramid is a type of bar graph that depicts the number or percentage of men and women in certain age cohorts in a given population. Expanding pyramids reflect growing populations with more young than old individuals; stationary pyramids reflect stable populations; and contracting pyramids reflect gradually declining populations.
Introductory Biology makes up 65% of the Biological and Biochemical Foundations of Living Systems section of the MCAT. Knowledge of genetic, molecular, and cellular processes is instrumental in understanding how tissues and organs function and form part of entire body systems. Try the free Biology practice question below:
Which pairs of substances are released from exocrine glands?
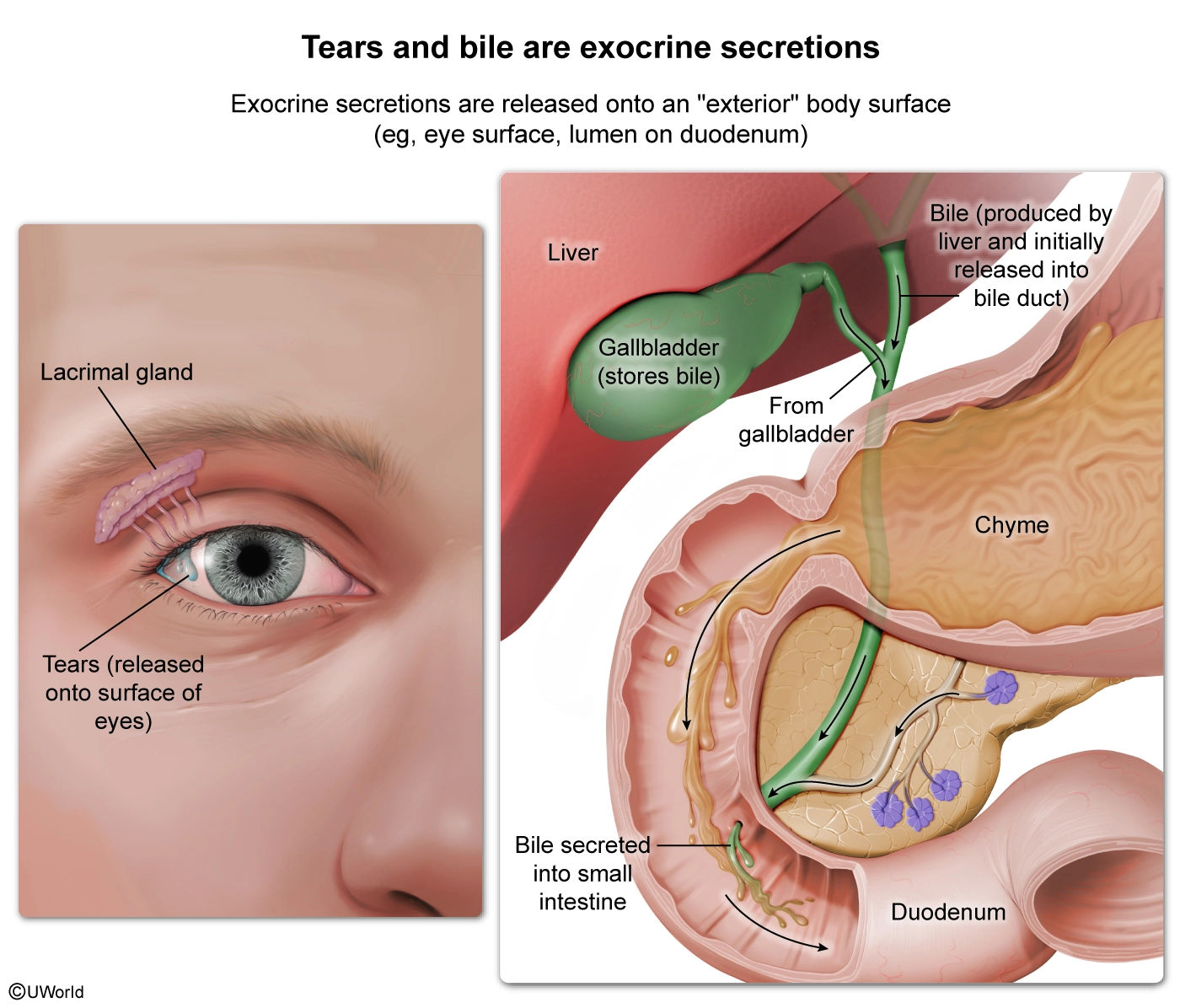
Certain types of epithelial cells are specialized for the secretion of substances into the extracellular space that surrounds them. Some of these secretory epithelial cells are solitary, being one of many epithelial cell types distributed across the surface of a tissue, whereas others are grouped together in a collection of secreting cells called a gland.
During development, some secretory cells form hollow tubes called ducts into which their secretions flow. For example, mammary glands secrete breast milk through ducts connected to the body’s exterior via the nipple, and sweat glands secrete sweat into ducts that empty onto the skin’s surface via pores. Glands whose secretions are released onto an “exterior” body surface (such as the skin or the inner surface of the lung) are called exocrine glands.
Other secretory epithelial cells develop around blood vessels but lack ducts; these cells release their secretions, called hormones, into the blood. Glands composed of cells such as these are called endocrine glands.
Some organs contain both endocrine and exocrine cells. For example, the pancreas secretes both digestive enzymes (an exocrine secretion) and insulin and glucagon, which are hormones (endocrine secretions).
The question asks for the pair of substances that would be secreted from exocrine glands. Tears are secreted onto the eye’s surface from lacrimal glands near the eyes, and bile is secreted from the liver into the bile duct, from which it is released into the duodenum of the small intestine (recall that the epithelial surfaces of the stomach, intestines, and lungs border cavities that are in communication with the exterior of the body). Therefore, tears and bile are both secreted from exocrine glands.
(Choices A, B, and C) Insulin and glucagon are hormones. Because hormones are secreted into the blood from endocrine glands, these choices are not examples of pairs of substances secreted from exocrine glands.
Educational objective:
Both endocrine and exocrine cells are epithelial cells that produce substances released into the extracellular space. Exocrine secretions are released to the body’s “exterior,” such as the skin or the inner surface of the gut or lungs. Endocrine secretions are released into the blood.
General Chemistry makes up 30% of the Chemical and Physical Foundations of Biological Systems section of the MCAT. Knowing basic principles in General Chemistry will help you understand matter and energy and how the two interact. Try the free General Chemistry sample question below:
2 Bi(s) + 5 F2(g) → 2 BiF5(s)
Metallic, elemental bismuth reacts with yellow fluorine gas to form a white solid of bismuth(V) fluoride according to the balanced equation above. How many grams of fluorine gas are needed to fully convert 836 g of bismuth in this reaction?
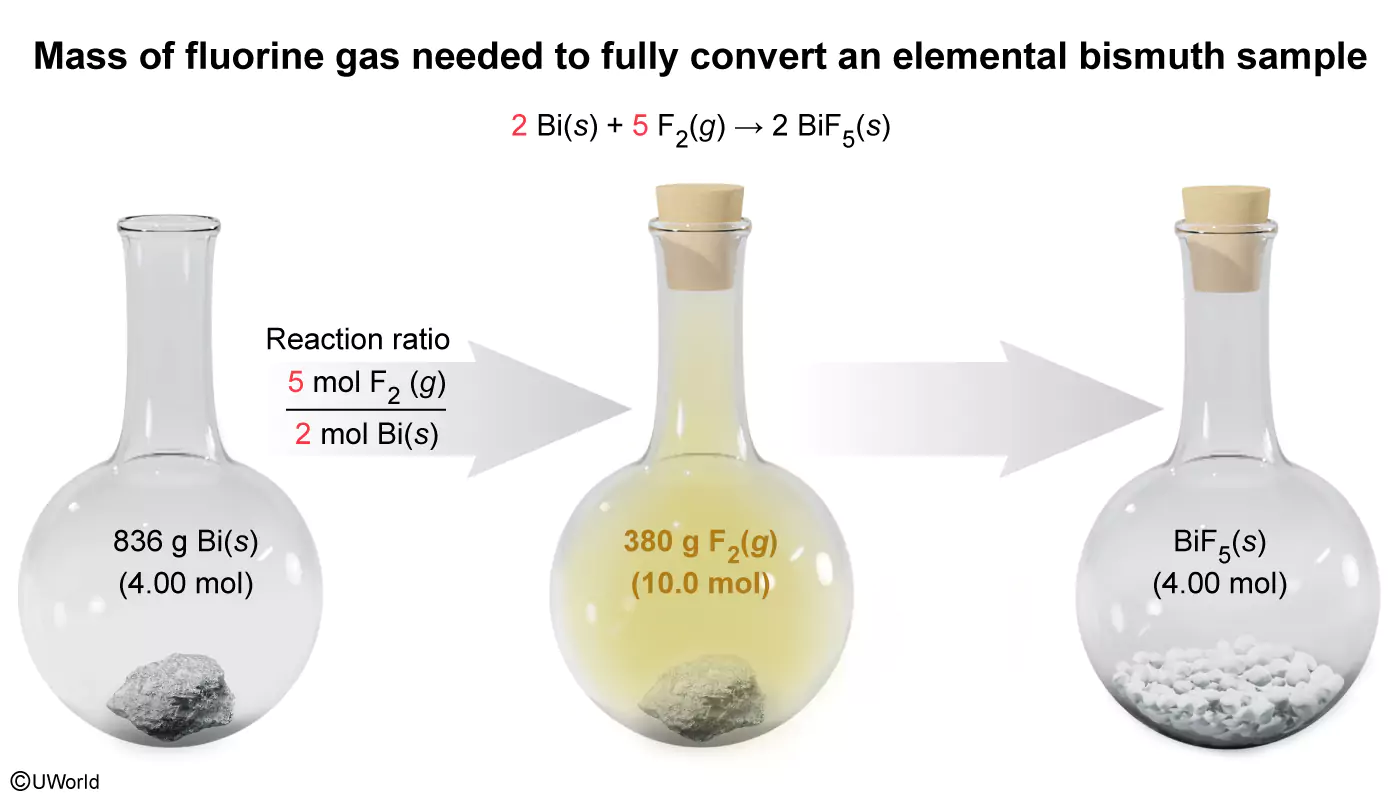
The molar mass of a compound is the amount of mass contained in 1 mole of the compound (ie, the grams per mole) and can be used to convert the mass of a compound to the equivalent number of moles. As such, the number of moles of Bi(s) in an 836 g sample can be found by dividing the mass of Bi(s) by its molar mass from the periodic table:
836The number of moles of a chemical species is related to the number of moles of another chemical species in a reaction by the mole ratios of the stoichiometric coefficients from a balanced reaction equation.The given balanced reaction equation shows that 5 mol of F2(g) are consumed for every 2 mol of Bi(s) that reacts. Applying this mole ratio, the number of moles of F2(g) needed to fully convert the Bi(s) sample in the reaction is:
4.00The mass of this number of moles of F2(g) can be found by multiplying the moles of F2(g) by its molar mass calculated from the periodic table (ie, molar mass F2(g) = 2 × 19.0 g/mol = 38.0 g/mol).
10.0(Choice A) A mass of 60.8 g results if the mole ratio 5 mol F2(g) / 2 mol Bi(s) is mistakenly inverted as 2 mol Bi(s) / 5 mol F2(g) during the second step of the calculation.
(Choice B) A mass of 152 g is obtained by incorrectly assuming a 1:1 mole ratio for F2(g):Bi(s) during the second step of the calculation.
(Choice C) A mass of 190 g is calculated if the molar mass of F (19.0 g/mol) is used instead of the molar mass of F2(g) (38.0 g/mol) in the last step of the calculation.
Educational objective:
The molar mass (the amount of mass in 1 mole of a compound) permits conversions between a given amount of mass and the number of moles that it contains. Stoichiometric mole ratios from a balanced reaction equation relate the number of moles of a chemical species to the moles of another chemical species in a reaction.
Organic Chemistry makes up 15% of the Chemical and Physical Foundations of Biological Systems section of the MCAT. Learning the principles behind chemical structures and organic material is a foundational step in understanding living systems. Try the free Organic Chemistry practice question below:
Consider the reaction below.
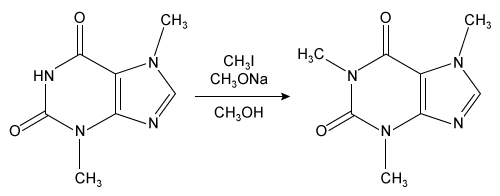
Which of the following observations would most likely indicate that the product from this reaction is pure?
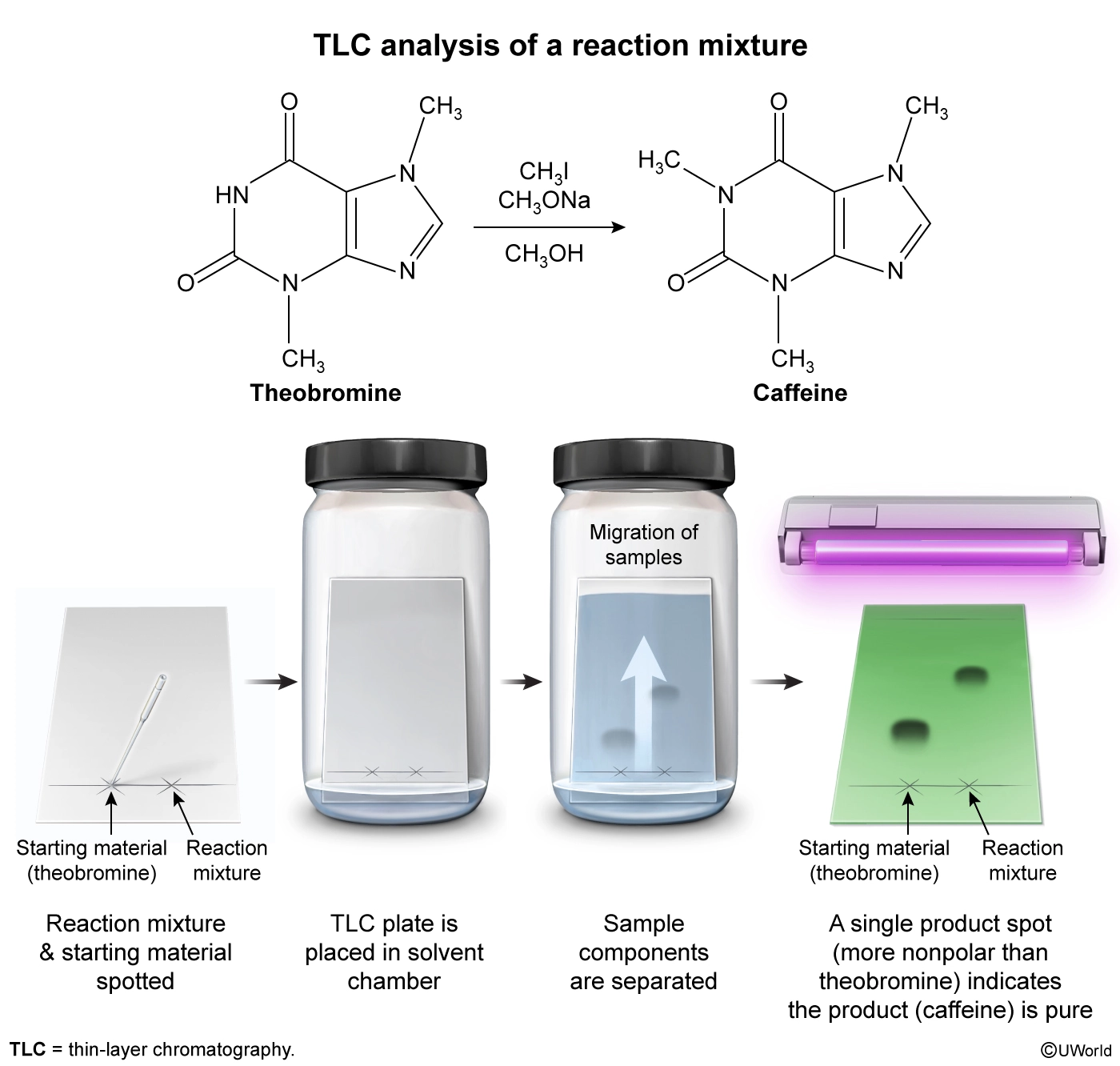
Several techniques can be used to evaluate the purity of a compound, including thin-layer chromatography (TLC), which separates compounds in a mixture based on polarity. In TLC, the tested sample is dissolved in an organic solvent and a small amount is spotted onto the stationary phase (commonly a polar silica [SiO2]) coated on a TLC plate. After spotting, the TLC plate is placed in a solvent chamber, and the mobile phase (organic solvent) travels up the plate and carries the spotted compounds along at different rates, which separates the mixture components. Nonpolar compounds have less affinity for a polar stationary phase and will travel further up the plate than polar compounds.
The conversion of theobromine to caffeine results in N-methylation (conversion of N–H to N–CH3), resulting in a large change in polarity. The N–H in theobromine can hydrogen bond to the polar stationary phase on the TLC plate, whereas the N–CH3 in caffeine cannot hydrogen bond. Because theobromine can hydrogen bond, it is more polar and has a smaller Rf value than caffeine, and it produces distinguishably different spots on a TLC plate. Therefore, TLC can be used to assess product purity by comparing a standard of pure theobromine to the isolated product. If a single spot with a larger Rf than that of theobromine is visible on the reaction mixture lane on the TLC plate, this indicates that the reaction proceeded to completion, no more reactant remained, and the isolated product is pure.
(Choice A) Size-exclusion chromatography separates compounds by size; therefore, compounds that are similar in size elute together. Theobromine and caffeine are very similar in size, differing only by a methylene group. Size-exclusion chromatography is unlikely to provide enough resolution to distinguish the two molecules; instead, they are likely to elute together and appear in one peak.
(Choice B) Although high-performance liquid chromatography (HPLC) can be used to determine product purity, a pure reaction product would show only one peak (not two) on the HPLC chromatogram.
(Choice C) There are three unique methyl (−CH3) groups in the reaction product caffeine; therefore, three unique −CH3 signals would be seen in its 1 H NMR spectrum. Two unique −CH3 signals would indicate the product had not formed because the reactant (ie, theobromine) has only two unique −CH3 groups.
Educational objective:
Thin-layer chromatography (TLC) is a technique used to evaluate a compound’s purity. The components of a mixture are separated based on polarity, and a single spot on a TLC plate is indicative of a compound’s purity.
Introductory Physics makes up 25% of the Chemical and Physical Foundations of Biological Systems section of the MCAT. Understanding the fundamentals of motion, electricity, magnetism, and similar topics will help you apply scientific reasoning in many medical situations. Try the free Physics sample question below:
A musician plays the trumpet in a marching band. The musician tunes the trumpet inside a room and then goes outside to play. When outside, the trumpet sounds flat (plays a lower frequency). Which of the following has occurred to account for the decrease in the frequency?
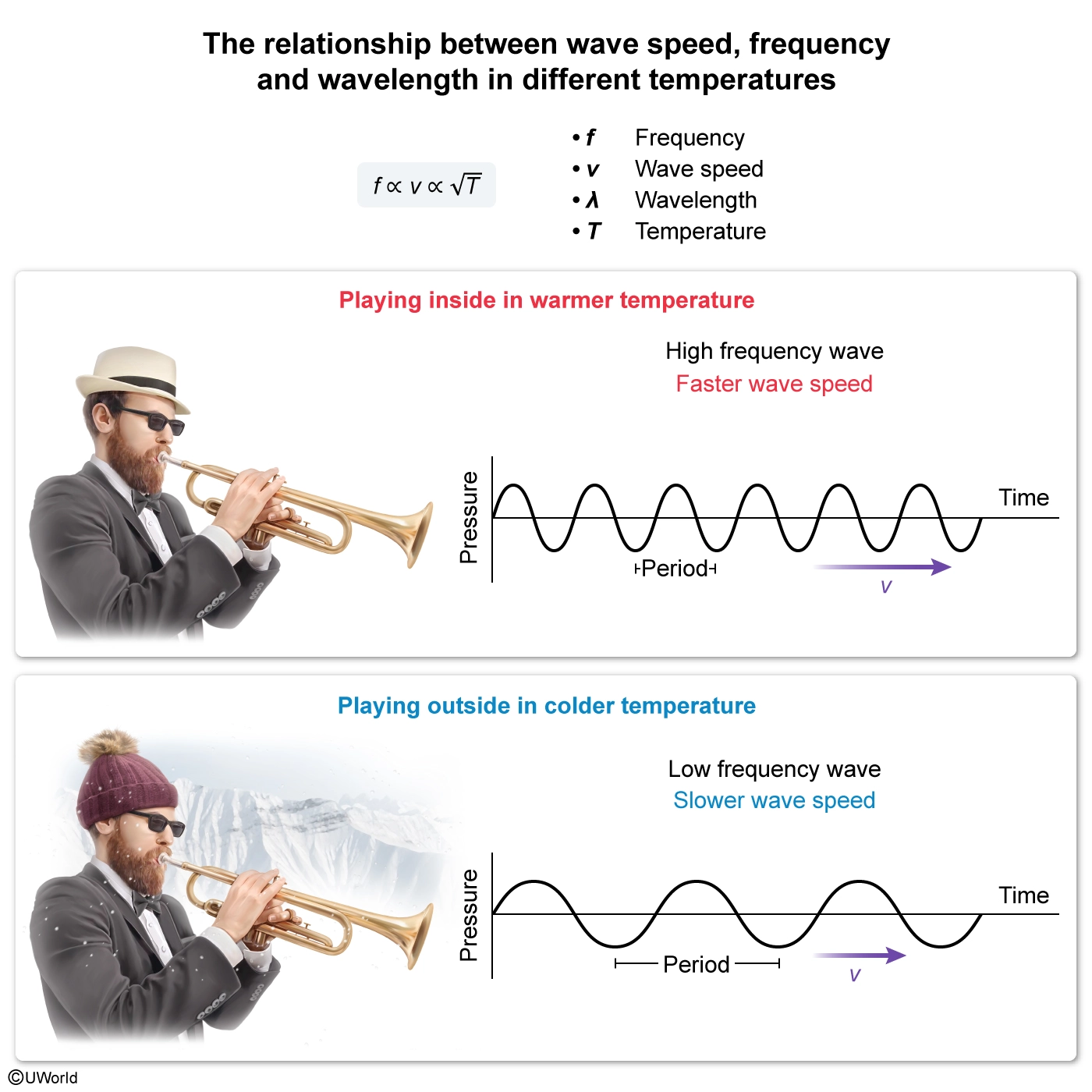
A wave is a motion created by a disturbance in a medium. A wave moves through the medium with a speed v that is related to the wavelength λ and frequency f:
v = λfRearranging, the frequency of a sound wave is directly proportional to v and inversely proportional to λ:
f = v / λThe speed of waves is determined by the physical properties of the medium through which they travel. For example, the speed of sound waves depends on the temperature of the medium: sound travels faster in warmer air and slower in cooler air. In particular, the speed of sound in air follows the following formula (in kelvin, K):
v = 331 m / s . √ T / 273KConsequently, when the wavelength is constant, an increase (or decrease) in wave speed results in a corresponding increase (or decrease) in the wave’s frequency.
In this question, the frequency of the trumpet’s sound is flat when the musician plays outside. Because the wavelength of the sound is fixed by the size of the tube, the decrease in frequency is caused by a decrease in v. Therefore, the decrease in frequency can be accounted for by the air outside being cooler than the air inside, which decreases the speed of the sound waves.
(Choice A) Sound waves travel faster in warmer air. Hence, the speed of sound waves increases, causing the frequency to increase, not decrease.
(Choice B) The energy of a wave is related to the wave’s intensity and loudness but not the wave’s frequency. Dissipation of energy does not affect the frequency of a wave.
(Choice C) The wavelength of a sound wave produced by a brass instrument depends on the length of the tube. A shorter trumpet results in a shorter wavelength and a higher frequency.
Educational objective:
The frequency of sound is directly related to wave speed and inversely related to wavelength. Sound waves travel faster in warmer air and slower in cooler air.
MCAT Critical Analysis and Reasoning Skills (CARS) passages are similar to standard verbal reasoning tests and require you to comprehend, analyze, and reason your way to solutions. They are designed to measure the reasoning and analysis skills you will need to succeed in medical school. Try the free CARS practice question below:
Locomotives were invented in England, with the first major railroad connecting Liverpool and Manchester in 1830. However, it was in America that railroads would be put to the greatest use in the nineteenth century. On May 10, 1869, the Union Pacific and Central Pacific lines met at Promontory Point, Utah, joining from opposite directions to complete a years-long project—the Transcontinental Railroad. This momentous event connected the eastern half of the United States with its western frontier and facilitated the construction of additional lines in between. As a result, journeys that had previously taken several months by horse and carriage now required less than a week’s travel. By 1887 there were nearly 164,000 miles of railroad tracks in America, and by 1916 that number had swelled to over 254,000.
While the United States still has the largest railroad network in the world, it operates largely in the background of American life, and citizens no longer view trains with the sense of importance those machines once commanded. Nevertheless, the economic and industrial advantages those citizens enjoy today would not have been possible without America’s history of trains; as Tom Zoellner reminds us, “Under the skin of modernity lies a skeleton of railroad tracks.” Although airplanes and automobiles have now assumed greater prominence, the time has arrived for the resurgence of railroads. A revitalized and advanced railway system would confer numerous essential benefits on both the United States and the globe.
The chief obstacles to garnering support for such a project are the current dominance of the automobile and the languishing technology of existing railroads. In a sense these two obstacles are one, as American dependence on personal automobiles is partially due to the paucity of rapid public transportation. The railroads of Europe and Japan, by comparison, have vastly outpaced their American counterparts. Japan has operated high-speed rail lines continuously since 1964, and in 2007, a French train set a record of 357 miles per hour. While that speed was achieved under tightly controlled conditions, it still speaks to the great disparity in railroad development between the United States and other countries since the mid-twentieth century. British trains travel at speeds much higher than those in America, where both the trains themselves and the infrastructure to support them have simply been allowed to fall behind. In much of Europe it is common for trains to travel at close to 200 miles per hour.
To invest in a modern network of railroads would improve the United States in much the same way that the first railroads did in the nineteenth and early twentieth centuries. A high-speed passenger rail system would dramatically transform American life as travel between cities and states became quicker and more convenient, encouraging commerce, business, and tourism. Such a system would also make important strides in environmental preservation. According to a 2007 British study, “CO2 emissions from aircraft operations are…at least five times greater” than those from high-speed trains. For similar reasons, Osaka, Japan, was ranked as “the best…green transportation city in Asia” by the 2011 Green City Index. As Lee-in Chen Chiu notes in The Kyoto Economic Review, Osakans travel by railway more than twice as much as they travel by car.
It is true that developing a countrywide high-speed rail system would come with significant costs. However, that was also true of the original Transcontinental Railroad, as indeed it is with virtually any great project undertaken for the public good. We should thus move ahead with confidence that the rewards will outweigh the expenditure as citizens increasingly choose to travel by train. Both for society’s gain and the crucial well-being of the planet, our path forward should proceed upon rails.